Saphenion®: Venenkleber 10 Jahre in der Vene?Venenkleber langfristig in der Vene nachweisbar?
Saphenion®: VenaSeal 10 years inside the veins? Is vein glue detectable in the vein in the long term?
Saphenion®: Venenkleber 10 Jahre in der Vene? In letzter Zeit häufen sich in den Medien und auf Webseiten Aussagen von Kollegen, die den Venenkleber sehr populistisch bewerten und dabei auch nicht vor Schreckensbegriffen, wie: “ Unmengen von Chemie“, “ Sekundenkleber“ und „Gifteinspritzungen in die Vene“ zurückschrecken. Dabei wird bewusst negiert, dass es weltweit seit Mitte der 60er Jahre auch theoretische Forschungen zur Gewebeverträglichkeit, zum biologischen Abbau und zur Wirkung des Klebers „Cyanoacrylat“ gibt und auch die amerikanische Gesundheitsbehörde FDA 2015 umfangreiche Prüfprotokolle zum Einsatz in den Krampfadern veröffentlicht hat. Abgesehen davon, dass eben diese Prüfung bereits 2011 auch in der EU durchgeführt worden war. Inzwischen hat die FDA den Venenkleber „VenaSeal“ 2018 in den USA zu einer Versicherungsleistung erklärt.
Lately, in the media and on websites phrases of colleagues who rate the venous adhesive very populist and do not shrink from horrors such as: „tons of chemistry“, „superglue“ and „poison injections into the vein“. It is deliberately denied that there has been worldwide research since the mid-sixties on the tissue compatibility, biodegradation and the effect of the adhesive „cyanoacrylate“ The US Food and Drug Administration FDA 2015 has published extensive test protocols for use in varicose veins. Apart from the fact that just this test had already been carried out in 2011 in the EU. In the meantime, the FDA has declared the vein adhesive „VenaSeal“ 2018 in the USA an insurance benefit.
Saphenion®: Venenkleber 10 Jahre in der Vene?Was ist dran an diesen Erklärungen?
Zum Einen sind inzwischen weltweit über 700 000 Patienten mit dem Kleber behandelt worden. Zum Zweiten werden je Stammkrampfader lediglich 1,5 – 1,8 ml des Klebers benötigt.
Dazu kommen – in regelmäßigen Abständen – ausgesprochen positive Erfahrungsberichte in der internationalen Fachpresse zu den klinischen – und Anwendungsergebnissen.
Die Basis des Venenklebers bildet – wie seit Beginn der 60er Jahre auch bei allen anderen operativen Einsatzgebieten – das Cyanoacrylat. Dieses wird jedoch chemisch so verändert und adaptiert, dass eine gute Gewebeverträglichkeit und eine Bioresorption erreicht werden.
Über die Bioresorption gut es inzwischen eine Reihe von Tierversuchen an Ziegen, Kaninchen und Albinoratten. Bisher fehlte allerdings der histopathologische Nachweis für die Bioresorption des Klebers in menschlichen Venen. Es wäre auch irre und ethisch nicht vertretbar, wenn man behandelte Patienten bitten würde, sich ein Stück der verklebten Vene zu Forschungszwecken entfernen zu lassen.
Saphenion®: VenaSeal 10 years inside the veins? What’s wrong with these explanations?
On the one hand, more than 700,000 patients worldwide have been treated with the vein glue. Secondly, only 1.5 – 1.8 ml of the glue is needed in the treatment of one truncal varicose vein.
In addition, there are – at regular intervals – very positive reports in the international scientific press on the clinical and application results.
The basis of the vein glue forms – as since the beginning of the 60s also in all other surgical areas – the cyanoacrylate. However, this is chemically altered and adapted for good tissue compatibility and bioresorption.
Bioresorption is good for several animal experiments on goats, rabbits, and albino rats. So far, however, lacked the histopathological evidence for the bioresorption of the adhesive in human veins. It would also be wrong and ethically unreasonable to ask treated patients to have a piece of the bonded vein removed for research purposes.
Saphenion®: Venenkleber 10 Jahre in der Vene?Eine eigene Fallbeschreibung zum Thema…
Vor 5 Jahren haben wir bei einer Patientin eine VenaSeal – Therapie simultan an beiden Stammvenen durchgeführt. Der post operative Verlauf verlief völlig unauffällig.
Nach 12 Monaten stellt sie sich dann mit einem ca. 1 cm grossen entzündeten Venenstrang der vormals verklebten Stammkrampfader am li. Oberschenkel vor. Nach zunächst konservativer Therapie mit entzündungshemmenden Medikamenten und Alkoholkompressionsverband entschieden wir uns nach 14 Tagen, den entzündeten Venenabschnitt nach guter alter Chirurgensitte mittels eines kleinen Einstichs zu entfernen. Wir entleerten den gesamten entzündeten Inhalt einschließlich der Venenreste und sandten diesen in ein Labor für Pathologie und Histologie. Natürlich gingen wir davon aus, dass in der Histologie auch Kleberbestandteile und – reste nachweisbar sein würden.
Saphenion®: VenaSeal 10 years inside the veins? A recent casuistry surprised us …
One year ago we performed VenaSeal therapy simultaneously on both truncal GSV in a female. The postoperative course was completely unremarkable.
After 12 months she then presents herself with a 1 cm varicose vein phlebitis of the formerly treated sealed vein on the left leg. After initially conservative therapy with anti-inflammatory drugs and an alcohol compression bandage we decided after 14 days to remove the inflamed venous section after a good old surgeon method using a small cut. We emptied all the inflamed contents, including the remains of the veins, and sent it to a laboratory for pathology and histology. Of course, we assumed that in histology also adhesive components and residues would be detectable.

Es hat uns dann doch überrascht, dass dies nicht der Fall war. Es wurden reichlich Entzündungszellen nachgewiesen, dazu Fibrin und elastische Fasern und Reste der Venenwand, jedoch kein Kleber oder andere Kunststoffe!
It surprised us then that this was not the case. There were plenty of inflammatory cells detected, plus fibrin and elastic fibers and remains of the vein wall, but no glue or other plastics!
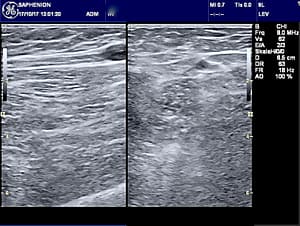
Saphenion®: Venenkleber 10 Jahre in der Vene? Ultraschallbild von der behandelten Vene mit dem entzündeten Abschnitt
Damit haben wir, eigentlich eher zufällig, erstmals am Menschen einen Beweis für den biologischen Abbau des Venenklebers in den Händen. Wir fühlen uns demnach ein wenig bestätigt in der Therapieoption Venenkleber, die wir neben der Radiowelle und dem Mikroschaum anbieten. Und wir freuen uns darauf, dass vielleicht jetzt etwas sachlicher und unvoreingenommener über dieses seit 2011 praktizierte Verfahren diskutiert werden kann.
Und ich lade die Zweifler gerne ein, ein kleines Stück einer bei mir selbst verklebten Vene zu entfernen, um diese dann auch untersuchen zu lassen.
Thus, for the first time on humans, we have, for the first time, proof of the biodegradation of the vein glue in our hands. We therefore feel a little confirmed in the treatment option venecylar, which we offer in addition to the radio wave and the microfoam. And we are looking forward to discussing this factual and unprejudiced procedure, which has been practiced since 2011, perhaps now.
And I invite the doubters to remove a small piece of my sealed vein

Photos: Utzius
Links/Papers:
3-Methoxybutylcyanoacrylate: evaluation of biocompatibility and bioresorption.
Author information
The biocompatibility and bioresorption of 3-methoxybutylcyanoacrylate (MBCA) was evaluated in vivo using female Wistar albino rats. MBCA was found to elicit slight to moderate tissue reaction similar to isobutylcyanoacrylate (iBCA) which has been sold commercially as a surgical adhesive (Bucrylate, Ethicon). MBCA was judged less reactive to tissue than ethylcyanoacrylate (ECA). The MBCA implants in rat gluteal muscles also resorbed within approx. 16 wk while iBCA implants remained essentially unchanged at 36 wk in vivo. In vitro resorption in phosphate buffer (pH 7.2) at 37 degrees C showed the same trend. The MBCA performed similarly to iBCA as a haemostat on excised rat livers and as an adhesive on rat skin incisions and had comparable adhesive bond strength.
Comparative study of suture and Cyanoacrylates in skin closure of rats.
Author information
PURPOSE:
To compare the biocompatibility of ethyl-cyanoacrylate (ECA) and octylcyanoacrylate (OCA) wound closures to sutures in rat skin.
METHODS:
Twenty-four male Wistar rats were subjected to three incisions which were closed using ECA, OCA or sutures . Rats were divided into four groups which received biopsies on the 3rd, 7th, 14th or 21st post-operative days. Necrosis, inflammation, dermatitis, infection, dehiscence, cicatricial enlargement and costs were examined; the histopathology evaluated was epithelialization, deep openings, foreign substance reaction, residues of synthesis material, fibrosis, inflammation, dehiscence and necrosis.
RESULTS:
The tissue adhesives presented the largest dehiscence levels, and ECA the lowest cost while the other measures were similar. Regarding histopathology, deep openings were more common with OCA and granulomas were most frequently obtained with ECA. The two tissue adhesives produces less inflammation than the inicial suture from post-operative day 7, while ECA and OCA cause similar inflammatory reactions. ECA did not differ significantly from OCA and sutures on other measures.
CONCLUSION:
ECA was well tolerated in this study and did not induce necrosis, allergic reactions or infections, presenting several advantages in relation to OCA and sutures, including lower costs and fewer complications.
A prospective, randomized, controlled clinical trial of tissue adhesive (2-octylcyanoacrylate) versus standard wound closure in breast surgery.
Abstract
BACKGROUND:
Recent studies suggest that the use of tissue adhesive for closure of both traumatic lacerations and incisional surgical wounds leads to cosmetic outcome comparable to conventional sutures. To date, no studies have investigated tissue adhesive in breast surgery and costs. Our aim was to compare the tissue adhesive 2-octylcyanoacrylate (OCA) with standard suture in breast surgery.
METHODS:
A prospective randomized study was conducted in which 151 patients were assessed for eligibility, and 133 were randomly allocated to skin closure with OCA adhesive or monofilament suture. Cosmetic outcome with blinded assessment, wound management by the patients, complication rates, and economic outcome were recorded.
RESULTS:
There was no difference in cosmetic score in the 2 groups, nor in complications at the early, 6-month, and 1-year follow-up. Patient satisfaction with the wound closed with OCA was rated significantly higher when compared with standard suture (P <.0001). The application of the tissue adhesive was significantly faster than that for standard suture (P <.001). In economic terms total costs were less in the tissue adhesive group, mainly due to lower postoperative costs of physician and assistant services (P <.001).
CONCLUSIONS:
OCA is effective and reliable in skin closure for breast surgery, yielding similar cosmetic results to standard suture. OCA is faster than standard wound closure and offers several practical advantages over suture repair for patients. Cost analysis has found that OCA adhesive can significantly decrease health care costs.
Comparing cyanoacrylate tissue adhesive and conventional subcuticular skin sutures for maxillofacial incisions–a prospective randomized trial considering closure time, wound morbidity, and cosmetic outcome.
Author information
PURPOSE:
To compare octyl-2-cyanoacrylate (2-OCA) tissue adhesive with subcuticular suture for the closure of incisions in the maxillofacial region to determine 1) whether it is faster than traditional subcuticular suturing, 2) whether the number and length of incisions affect closure time, 3) wound morbidity, 4) patient satisfaction outcome, and 5) cosmetic outcome.
MATERIAL AND METHODS:
In a prospective randomized clinical trial, 29 patients were allocated to 1 of 2 groups for the closure of skin incisions using 2-OCA or conventional subcuticular skin sutures. Postoperative follow-up evaluated wound healing at 5 to 10 days and at 3 months. Assessment of cosmetic outcome was performed by a plastic surgeon using a modified Hollander Wound Evaluation Scale and a validated visual analog scale. Comparisons between groups were performed using the Student t test and χ(2) test.
RESULTS:
Twenty incisions in 14 patients were closed with 2-OCA and 20 incisions in 15 patients were closed with subcuticular sutures. Mean time of closure was significantly (P < .005) faster with 2-OCA at 69.50 ± 33.39 seconds compared with 379.00 ± 75.39 seconds in the suture group. There was no significant difference in wound complications between the 2 groups; also, there was no significant difference in patient satisfaction and cosmetic outcome of scars at the 3-month follow-up between the 2 groups.
CONCLUSIONS:
2-OCA tissue adhesive is an excellent alternative to sutures for effective, reliable, and faster skin closure of maxillofacial incisions.
Copyright © 2013 American Association of Oral and Maxillofacial Surgeons. Published by Elsevier Inc. All rights reserved.
Neurosurgical applications of the cyanoacrylate adhesives.
Abstract
The cyanoacrylate adhesives are a biologically heterogenous group, some of which are potentially valuable additions to the neurosurgical armamentarium. The commercially available cyanoacrylates usually contain the more toxic methyl and ethyl monomers. The safe butyl monomer, isobutyl 2-cyanoacrylate, is approved in the United States by the Food and Drug Administration for investigational use only. Isobutyl 2-cyanoacrylate may prove to be useful for the extravascular reinforcement of intracranial aneurysms and for the intravascular occlusion of carotid-cavernous fistulae. Safe and effective alternatives exist for the management of these two problems. The sealing of certain cerebrospinal fluid fistulae and the intravascular occlusion of certain arteriovenous malformations may be more effectively accomplished with isobutyl 2-cyanoacrylate than with other currently available techniques. The ultimate role of this and of other as yet untested cyanoacrylates in neurosurgery remains to be determined.
Variceal bleeding in the small intestine successfully treated with balloon-occluded retrograde transvenous obliteration using N-butyl-2-cyanoacrylate: A case report.
Abstract
Rupture of small intestinal varices associated with portal hypertension can be a serious condition that is difficult to diagnose early and to manage. Moreover, optimal guidelines for the treatment of small intestinal varices have not yet been established. We herein report a case of a 73-year-old man with small intestinal varices. The man presented with bleeding from a stoma in the small intestine, which subsequently led to hemorrhagic shock. We successfully treated the patient with balloon-occluded retrograde transvenous obliteration via the right inferior epigastric vein using N-butyl-2-cyanoacrylate.
KEYWORDS:
Balloon-occluded retrograde transvenous obliteration; N-butyl-2-cyanoacrylate; Small intestinal varices
Biocompatible alkyl cyanoacrylates and their derivatives as bio-adhesives.
Abstract
Cyanoacrylate adhesives and their homologues have elicited interest over the past few decades owing to their applications in the biomedical sector, extending from tissue adhesives to scaffolds to implants to dental material and adhesives, because of their inherent biocompatibility and ability to polymerize solely with moisture, thanks to which they adhere to any substrate containing moisture such as the skin. The ability to tailor formulations of alkyl cyanoacrylate to form derivative compounds to meet application requirements along with their biodegradability in conjunction with their inherent biocompatibility make them highly sought after candidates in the biomedical sector. There has been extensive exploration of cyanoacrylate adhesives and their homologue systems in biomedical applications, but no consolidated literature of the vast data is available. The ability of cyanoacrylate adhesives to cure at low temperatures and without the need for any hardener, which is attributed to the high-strength bonding interaction between two non-amalgamating substrates, with their ease of dispersion and self-curing, avoids the curtailing of the effective utilization of such adhesives in biomedical engineering applications as bio glues for amalgamating tissues, implants, scaffolds etc. This article consolidates copious work on cyanoacrylateadhesives and their derived systems which are functional in versatile biomedical engineering applications such as bio glues, dental material and adhesives and other potential applications.
Sutures versus new cyanoacrylates in prosthetic abdominal wall repair: a preclinical long-term study.
Abstract
BACKGROUND:
As an alternative to sutures, meshes used for hernia repair can be fixed using cyanoacrylate-based adhesives. Attempts to improve these adhesives include alkyl-chain lengthening to reduce their toxicity. This preclinical study compares the long-term behavior of cyanoacrylates of different chain lengths already used in hernia repair and new ones for this application.
MATERIALS AND METHODS:
Partial abdominal wall defects were repaired using a Surgipro mesh in 18 New Zealand White rabbits, and groups were established according to the mesh fixation method: sutures (control), Glubran 2 (n-butyl), Ifabond (n-hexyl), and the new adhesives SafetySeal (n-butyl), and Evobond (n-octyl). Six months after surgery, recovered implants were examined to assess adhesive degradation, host tissue reaction, and biomechanical strength.
RESULTS:
All the cyanoacrylate groups showed good host tissue incorporation in the meshes. Macrophage responses to Glubran and Ifabond were quantitatively greater compared with sutures. Cell damage caused by the adhesives was similar, and only Glubran induced significantly more damage than sutures. Significantly lower collagen 1/3 messenger RNA expression was induced by Ifabond than the remaining fixation materials. No differences were observed in collagen expression except slightly reduced collagen I deposition in Glubran/Ifabond and collagen III deposition in the suture group. Mechanical strengths failed to vary between the suture and cyanoacrylate groups.
CONCLUSIONS:
All cyanoacrylates showed good long-term behavior and tolerance irrespective of their long or intermediate chain length. Cyanoacrylate residues persisted at 6 mo, indicating their incomplete degradation. Biomechanical strengths were similar both for the adhesives and sutures.
Copyright © 2017 Elsevier Inc. All rights reserved.
Sutures versus new cyanoacrylates in prosthetic abdominal wall repair: a preclinical long-term study.
Author information
BACKGROUND:
As an alternative to sutures, meshes used for hernia repair can be fixed using cyanoacrylate-based adhesives. Attempts to improve these adhesives include alkyl-chain lengthening to reduce their toxicity. This preclinical study compares the long-term behavior of cyanoacrylates of different chain lengths already used in hernia repair and new ones for this application.
MATERIALS AND METHODS:
Partial abdominal wall defects were repaired using a Surgipro mesh in 18 New Zealand White rabbits, and groups were established according to the mesh fixation method: sutures (control), Glubran 2 (n-butyl), Ifabond (n-hexyl), and the new adhesives SafetySeal (n-butyl), and Evobond (n-octyl). Six months after surgery, recovered implants were examined to assess adhesive degradation, host tissue reaction, and biomechanical strength.
RESULTS:
All the cyanoacrylate groups showed good host tissue incorporation in the meshes. Macrophage responses to Glubran and Ifabond were quantitatively greater compared with sutures. Cell damage caused by the adhesives was similar, and only Glubran induced significantly more damage than sutures. Significantly lower collagen 1/3 messenger RNA expression was induced by Ifabond than the remaining fixation materials. No differences were observed in collagen expression except slightly reduced collagen I deposition in Glubran/Ifabond and collagen III deposition in the suture group. Mechanical strengths failed to vary between the suture and cyanoacrylate groups.
CONCLUSIONS:
All cyanoacrylates showed good long-term behavior and tolerance irrespective of their long or intermediate chain length. Cyanoacrylate residues persisted at 6 mo, indicating their incomplete degradation. Biomechanical strengths were similar both for the adhesives and sutures.
Copyright © 2017 Elsevier Inc. All rights reserved.
KEYWORDS:
Abdominal wall repair; Cyanoacrylates; Mesh fixation; Polypropylene
Almeida JI, Murray SP, Romero ME. Saphenous vein histopathology 5.5 years after cyanoacrylate closure. J Vasc Surg Venous Lymphat Disord. 2020 Mar;8(2):280-284. doi: 10.1016/j.jvsv.2019.04.014. Epub 2019 Jul 4. PMID: 31281102.
Bahi M, Guazzo L, Taumoepeau L. Real-world short-term VenaSeal ablation outcomes for symptomatic saphenous incompetence. Vascular. 2023 Jun;31(3):521-525. doi: 10.1177/17085381221077511. Epub 2022 Feb 25. PMID: 35209758.
Gibson K, Ferris B. Cyanoacrylate closure of incompetent great, small and accessory saphenous veins without the use of post-procedure compression: Initial outcomes of a post-market evaluation of the VenaSeal System (the WAVES Study). Vascular. 2017 Apr;25(2):149-156. doi: 10.1177/1708538116651014. Epub 2016 Jul 9. PMID: 27206470.
Park I. Human Saphenous Vein Histopathology 2 Years After Cyanoacrylate Closure Using the VenaSeal™ System. Ann Vasc Surg. 2021 Feb;71:534.e17-534.e21. doi: 10.1016/j.avsg.2020.09.017. Epub 2020 Sep 16. PMID: 32949737.
Tang TY, Yap CJQ, Chan SL, Soon SXY, Yap HY, Lee SQW, Choke ETC, Chong TT. Early results of an Asian prospective multicenter VenaSeal real-world postmarket evaluation to investigate the efficacy and safety of cyanoacrylate endovenous ablation for varicose veins. J Vasc Surg Venous Lymphat Disord. 2021 Mar;9(2):335-345.e2. doi: 10.1016/j.jvsv.2020.03.020. Epub 2020 May 7. PMID: 32387378.
