Saphenion®: 7 years experience – Sealing 2359 truncal veins
Our long term experience in the sealing of varicose veins with the vein super glue VenaSeal be updated again:
In the period from 1st August 2012 to 17st. August 2019 (7 years) vein glue used on 2359 truncal varicose veins in 1250 patients. In 15 patients a leg ulcer (open leg) could be completely healed within 2 – 12 weeks. The vein glue has also been used in cases of dementia, multiple sclerosis or early childhood debility as well as in immunosuppression (HIV) and hepatitis patients. Even in 5 patients with multiallergy disposition the VenaSeal glue could be used successfully and without any side effects. Even with phlebitis until 2 weeks before the therapy date, we successfully used the vein glue.
In 148 cases (11,8%), our patients deliberately avoided any local anesthetic or sedative medication.
Non tumescent non thermal – 7 years vein glue
Since 8/2012 we use the vein glue at Saphenion. First use parallel to radiofrequency therapy. Meanwhile, more than 95% of our patients choose the glue for the treatment of truncal varicose veins.
In the meantime, technical indications have broadened the indications. Now we are treating for recurrent episodes, venous tumors, vein ectasies and aneurysms as well as perforating veins.
In particular, in strong and obese patients, we see clear advantages of the catheter method radio wave and especially the non tumescent non thermal vein glue – we have been successful in many cases in this group of patients with the vein glue therapy. In the meantime, we reject a classic stripping operation in the obese patient due to the very large op – areas and correspondingly high number of side effects.
Saphenion®: 7 years experiences – Follow up since 8/2012
All patients undergo duplex sonographic follow – up examinations. This serves above all the own quality control and the collection of experiences in this relatively new technology. The follow – up examination takes place on the 1st day, on the 14th – 30th day and between 90 and 120 days after the sealing. Afterwards we examine after half a year and then once a year. In recent months, we have focused our treatment regime on the simultaneous treatment of all truncal varicose veins in a single session. This provides a further significant gain in comfort for the patient. This results in considerable cost savings for private insurance carriers, assistance agencies and even the self – payers.
Saphenion®: 7 years experience – Our results
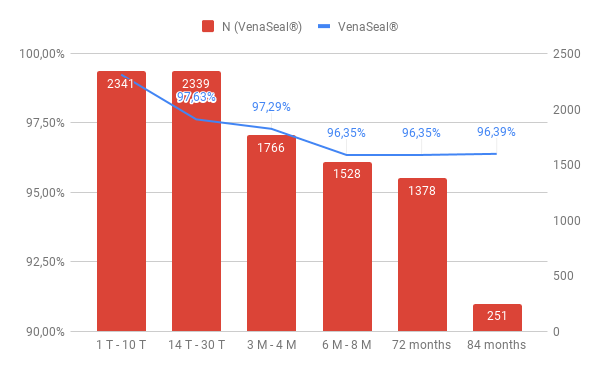
On the 1st day, all 2359 sealed veins were re-examined. The closure rate is 99.24%.
By the 30th. day we found a partial recanalization in 44 truncal veins and a complete recanalization in 12 cases. This corresponds to a closure rate of 97.63%.
Over 3 – 4 months, we observed 45 partially recanalized and 19 fully recanalized veins, with a closure rate of 97.29%.
After 6 – 8 months, we found 54 partially recanalized and 32 fully recanalized truncal veins after sealing (96.39%).
Further recanalizations were not found over the observation period. The closure efficancy of the vein glue in our single – center study is 96.39% after a long – term period of 7 years.
Patients usually do not need a compression stocking after the surgery.
Already on the 1st. post op day could be started with the usual sport – also competitive sports – in certain stress levels (except weight training – here we recommend over 14 days waiver of leg press f.e.). Also sauna sessions are possible again after 4 – 5 days – here are amazing subjective positive effects – a much higher heat tolerance and the absence of any complaints are described.
For compression stocking therapy, we recommend sealing veins only when strongly dilated veins from 1.2 cm in diameter and venous extensions or aneurysms have been treated.
Saphenion®: 7 years experience – Side effects
In 7,8% of all cases we saw a nonspecific foreign body reaction develops (tissue redness and swelling). This is indicated by a local reddening along the treated vein or as a planar reaction. However, this is not phlebitis, as is often misdiagnosed.
Pigmentation developed in 3% of the cases treated by us. This is especially in cases where the truncal vein was located just under the skin outside the normal anatomy.
In 22 patients there was a slight bleeding tendency of the puncture site. All of these patients did not have to discontinue their anticoagulant medication under VenaSeal – therapy (e.g., Marcumar, Falithrom, ASS, Aspirin, Plavix, Xarelto a.s.o.).
In 9 cases (0,7%) we saw a lymph – fistula at the puncture site, which healed after 14 – 21 days compression bandage.
A (glue?) – pimple with opening of the skin was found in 3 patients between 10 and 12 months after the therapy. These were emptied minimally invasively and then healed.
The pathological examination revealed that no glue residues were found!
We did not see any allergy or hematoma, no sensation, no numbness or other neurological side effects. Likewise, no phlebitis by the glue, no deep vein thrombosis or pulmonary embolism. Postoperative swelling was extremely rare, short – term lymphedema is possible.
There was no thrombosis in all 1250 cases.
In 14 cases, after the therapy with the vein glue, we saw a glue nose protruding into the pelvic or knee vein. An anti – thrombosis therapy for 3 days made these glue nose disappear in all cases.
We did not see any permanent skin alteration or pigmentation or other side effects and complications known from venous surgery.
Saphenion: 7 years experiences – Discussion
Over a period of now 7 years sealing of varicose veins we achieved a closure efficiency of 96.35%. These are almost the same results as in the current multi – center study „VeClose – study“. This was published in late April 2017. The colleagues presented a closure rate of 96.8% in the sealing of truncal varicose veins over a study period of 3 years.
The results are therefore almost identical after 3 years, as well as after nearly 7 years of use. These are the first single – center studies beyond the required 3 years of scientific control. The occlusive effectiveness of the vein glue is slightly better than in current long – term studies of the radio frequency ablation.
International Scientific Recommendations
We are currently following the European Guidelines for the Use of Endovenous Op Techniques (ESVS 2015) – the gold standard that recommends the use of catheters for the treatment of common varicose veins and includes the US guidelines and guidelines. Thus, all colleagues who work with endovenous techniques worldwide have analog and comprehensible quality criteria for the respective endovenous therapy.
And some weeks ago also the German guidelines of varicose vein therapy (DGP) have including all endovenous techniques, also the vein glue, as a recommendation for therapy of truncal varicose veins.
Saphenion®: 7 years experiences – Non thermal ablation at SAPHENION 1st. choice
All endovenous procedures have many advantages over the radical surgical therapy and show not only good functional but also much better cosmetic results.
The complication rate is significantly reduced. Side effects are much rarer!
In the meantime, in our hospital, the sealing of varicose veins is the therapy of choice for the treatment of truncal varicose veins.
On the lower leg, the vein glue is in any case preferable to the laser and radio wave and superheated thermal methods, in order to avoid nerve damage ( – 40%).
Anesthesia or tumescent anesthesia is not necessary in any case, a sedation or local anesthesia of the puncture site is quite sufficient. On the contrary, 11,8% of patients have renounced all anesthesia.
The closure rate of the vein glue is comparable to or higher than that of the radio wave – but we also see significantly less pain and side effects.
Even in obese patients, the use of vein glue is very effective and gentle. Here we do without a recommendation for the radical „Stripping „.
With the modified application of the vein glue, numerous possibilities arise for carrying out more differentiated treatment strategies.
Saphenion®: 7 years experience – what our problems with VenaSeal?
•VenaSealwas approvedin 2011 in Europe – since first approva lthere was no change of catheters or glue or treatment protocol.
•After the first approval protocol there is not to be found another therapy procedure. So we have to change the technique to get guideline results by ourselves.
•VenaSeal is safe in the treatment of all truncal veins, side branches, and perforator veins until a diameter of 1,5 – 2 cm. Higher diameters are possible! But- why there is not to be behind a shorter catheter, a perforator catheter, an SSV catheter, or a sealing needle? Why do we need an expensive Teflon catheter?
•VenaSeal ist the first choice in the treatment of truncal veins of the lower leg – but why there is- since 2011 – the same unchanged glue quantity inside the glue ampoule? In normal You have to throw the rest of the glue away…!
•VenaSeal is the much expensive of all endovenous techniques, thats why therapy of more than one truncal vein simultaneously is recommendable – we can treat 3 – 4 truncalveins! But why we have to pay the same price for a catheter-system developed from 2003 to 2010?
Weblinks / papers:
Almeida JI, Min RJ, Raabe R, McLean DJ, Madsen M.: Cyanoacrylate adhesive for the closure of truncal veins: 60-day swine model results. Vasc Endovasc Surg 2011; 45: 631-635
Almeida JI, Mackay EG, Bautista C, Proebstle T.: Cyanoacrylate glue great saphenous vein ablation: preliminary 180 – day follow up of a first -in- man feasibility study of a no-compression-no-local-anaesthesia technique. J.Vasc Surg 2012; 55:297
Almeida, JI, Julian J. Javier, Ed Mackay, Claudia Bautista, Thomas M. Proebstle: One – Year follow up of first hand use of cyanoacrylate adhesive for treatment of saphenous vein incompetence: epub 20. dec. 2012
Creton D, Rea B, Pittaluga P, Chastanet S, Allaert FA. Evaluation of the pain in varicose vein surgery under tumescent local anaesthesia using sodium bicarbonate as excipient without any intravenous sedation. Phlebology 2011. (Epub ahead of print 21 November 2012)
Elias S, Raines JK. Mechanochemical tumescentless endovenous ablation: final results of the initial clinical trial. Phlebology
Huisman LC, Bruins RMG, van den Berg M, Hissink RJ. Endovenous laser ablation of the small saphenous vein: prospective analysis of 150 patients, a cohort study. Eur J Vasc Endovasc Surg 2009;38:199–202
Ivanova, Patricija: Post procedere neuropathy: comparison of surgery, EVLA and glue; Presentation on 2nd. NEEF, Riga, 17th.August 2019.
Keel D, Goldman MP. Tumescent anaesthesia in ambu- latory phlebectomy: addition of epinephrine. Dermatol Surg 1999;25:371-2
Lawson J, S Gauw, C van Vlijmen, P Pronk, M Gaastra, M Mooij, C Wittens: Sapheon: the solution? Phlebology 2013;0:1-8
Min RJ, Almeida JI, McLean DJ, Madsen M, Raabe R.: Novel vein closure procedere using a proprietary cyanoacrylate adhesive: 30-day swine model results. Phlebology 2012; epub jan. 2012
Morrison, Nick and Kathleen Gibson: Veclose Study: Preliminary Month 1 Data; 2nd Annual Cyanoacrylate Embolization Symposium, Mainz, Jan. 18, 2014
Neff, P.: Erfahrungen mit viskösem Cyanoacrylat in der Niederlassung – Patientenauswahl und Ergebnisse; vasomed 1, 2019; 31 – 33
Proebstle TM, Vago B, Alm J, Göckeritz O, Lebard C, Pichot O. Treatment of the incompetent great saphenous vein by endovenous radiofrequency powered segmental thermal ablation: first clinical experience. J Vasc Surg 2008;47:151–6.
Proebstle TM, Alm J, Rasmussen L, Dimitri S, Lawson JA , Whiteley M , Franklin IJ , Davies AH:The European Multicenter Study On Cyanoacrylate Embolization Of Refluxing Great Saphenous Veins Without Tumescent Anaesthesia And Without Compression Therapy. Abstract presented to the American Venous Forum Annual Meeting 2013, Phoenix ( AZ) USA
Proebstle TM, Alm J, Rasmussen L, Dimitri S, Whiteley M , Franklin IJ , Davies AH: Cyanoacrylate Adhesive For Treatment of Great Saphenous Vein Incompetence without Tumescent Anesthesia and without Compression Therapy, Abstract presented to the American College of Phlebology Annual Meeting 2012 Hollywood, Florida.
Rasmussen LH, Bjoern L, Lawaetz M, Lawaetz B, Blemings A, Eklof B.: Randomised clinical trial comparing endovenous laser ablation with stripping of the great Saphenous vein: clinical outcome and recurrence after 2years. Eur J Vasc Endovasc Surg 2010;39:630 – 35
Rosen RJ, Contractor S: The use of cyanoacrylate adhesives in the management of congenital vascular malformations. Sem Interv Radiol 2004; 21:59-66
Schäffer, N. et al.: Appositionsthrombus al Komplikation endovenöser Therapieverfahren; Phlebologie 2/2018; 93 – 101
Shadid N, Ceulen R, Nelemans P, et al. Randomized clinical trial of ultrasound-guided foam sclerotherapy versus surgery for the incompetent great saphenous vein. Br J Surg 2012;99:1062 – 70
Shepherd AC, Gohel MS, Lim CS, Hamish M, Davies AH. The treatment of varicose veins: an investigation of patient preferences and expectations. Phlebology 2010;25:54 – 65
Thum, J: Single Center Erfahrungen aus 234 Stammvenenbehandlungen mit viskösem n – Butyl – 2 – Cyanoacrylat- Verwendbarkeit, Akzeptanz, Ergebnisse; vasomed 1, 2019; 28 – 31
Üdris, Ints: 6 years single center results „Baltic Vein Clinic“ of truncal varicose vein sealing; Presentation on 2nd. NEEF, Riga, 17th. August 2019.
Wade, Darren: The evolution of superficial venous management has moved away from thermal techniques; Presentation on 2nd. NEEF, Riga, 17th. August 2019.
This is a topic which is near to my heart… Many thanks!
Exactly where are your contact details though? Hey there!
Someone in my Facebook group shared this website with us so
I came to give it a look. I’m definitely loving
the information. I’m book-marking and will be tweeting this to my followers!
Fantastic blog and wonderful design. It’s perfect time to make some plans for the
future and it is time to be happy. I’ve read this publish and if I may I desire to suggest you few interesting things or suggestions.
Maybe you can write subsequent articles regarding this
article. I wish to learn even more things approximately it!
http://www.cspan.net
of course, it`s possible…greetings