Saphenion®: Long-term results of vein glue – experience in the therapy of 4716 varicose veins
Saphenion®: We have published long-term results of vein glue in regular succession for 10 years. It aims to compare international publications on the subject with our own experiences and remain meaningful. Our patients are now very well informed about this therapy method for varicose veins, which has been offered for 14 years.
After 155 months of use, we have treated 2243 patients on 4716 saphenous veins – over 1,1 Mio. Patients worldwide have now been treated with the vein glue VenaSeal®. In the meantime, we have successfully used the vein glue VenaSeal® to clean the vein system in 67 patients with COVID-19 infection after they recovered.
Of course, slightly adapted hygiene measures in the operating room and practice are necessary. However, these are kept within practical limits in an outpatient specialist practice that works surgically and with catheter medicine since the hygiene status is already primarily increased.
https://www.ajsccr.org/article-details.php?tit=AJSCCR-v5-1792
https://www.ajsccr.org/uploads/IMG_510052.pdf
Saphenion®: Long-term results of vein glue – News from the German Specialist Association for Vein Medicine
Saphenion: Long-term results of vein glue—The German Society for Vein Medicine (DGP) also recommends therapy with the vein glue VenaSeal® for the treatment of truncal varicose veins in the current guidelines „S2k—guideline for diagnosis and therapy of varicosis.“
Together with colleagues from Munich, Hildesheim, Cologne, and Düsseldorf, Saphenion® Berlin / Rostock discussed and founded a „VenaSeal® – network“ in spring 2020 on the occasion of the „Bonn Vein Days“. We aim to summarize the therapy results with the vein glue, exchange experiences, communicate them to our patients, and become scientifically active. In November 2020, our joint „German Multicenter Study VenaSeal®“ was published.
Saphenion: long-term results vein glue – 155 months
Saphenion: Long-term results vein glue – 155 months ago, we used vein glue to treat varicose veins. There are now 4716 treated veins in the surgery book. We have used vein glue in 2243 patients so far. In addition to many well-informed patients who are now directly requesting this therapy method, statutory health insurance companies have also recognized the procedure as no longer so new in some cases.
Most private insurance companies have also accepted the therapy option of vein gluing varicose veins as part of the cost estimates presented. Likewise, according to reports from our patients, the federal aid offices, the states of Berlin / Brandenburg, Mecklenburg, Saxony / Anhalt, Saxony, and Thuringia, as well as Lower Saxony and Schleswig-Holstein, are ready to cover the costs of this therapy method, which was approved in Europe in 2011. Our patients should speak to their insurance company before arranging a therapy appointment if in doubt.
Saphenion® – our 2000th VenaSeal® – Patient
Saphenion®: Long-term results of vein glue
Saphenion®: long-term results of vein glue – After nearly 13 years of practical work with vein glue, we see, like the international studies, its high effectiveness with a very low rate of side effects. Initially approved and used by the manufacturer exclusively for normal-caliber saphenous veins, the glue has conquered other application areas in endovenous therapy. Ecstatic veins and aneurysms are now successfully treated with vein glue.
This becomes clear in the literature analysis. Colleagues from the USA, Korea, the Netherlands, Russia, and Hong Kong have presented 3-5-year studies. In addition to our 10-year long-term research and the German VenaSeal® multicenter study, Morrisson et al. presented a 5-year study, as did Gibbson et al. The results are almost identical in all studies. The closure rate after these long periods is between 94% and 97%.
Our results after 155 months of use are a 96,29% closure rate.
This means the vein glue is more effective than the established radio wave system, but has significantly fewer side effects and a shorter post-op recovery phase. The ability to work is restored on the same day, approximately three hours after the therapy, or the following day if sedation is used.
Gibson K, Morrison N, Kolluri R, Vasquez M, Weiss R, Cher D, Madsen M, Jones A. Twenty-four-month results from a randomized cyanoacrylate closure versus radiofrequency ablation trial for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2018 Sep;6(5):606-613. doi: 10.1016/j.jvsv.2018.04.009. Epub 2018 Jun 15. PubMed PMID: 29914814.
Tang TY, Yap CJQ, Chan SL, Soon SXY, Yap HY, Lee SQW, Choke ETC, Chong TT. Early results of an Asian prospective multicenter VenaSeal real-world postmarket evaluation to investigate the efficacy and safety of cyanoacrylate endovenous ablation for varicose veins. J Vasc Surg Venous Lymphat Disord. 2021 Mar;9(2):335-345.e2. doi: 10.1016/j.jvsv.2020.03.020. Epub 2020 May 7. PMID: 32387378.
Cho S, Park HS, Lee T, Byun SJ, Yun WS, Yang SS, Kim H, Kim WS, Joh JH, Jung IM. CASS (CyanoAcrylate closure versus Surgical Stripping for incompetent saphenous veins) study is a randomized controlled trial comparing clinical outcomes after cyanoacrylate closure and surgical stripping for treating incompetent saphenous veins. Trials. 2020 Jun 3;21(1):460. doi 10.1186/s13063-020-04393-0. PMID: 32493398; PMCID: PMC7268719.
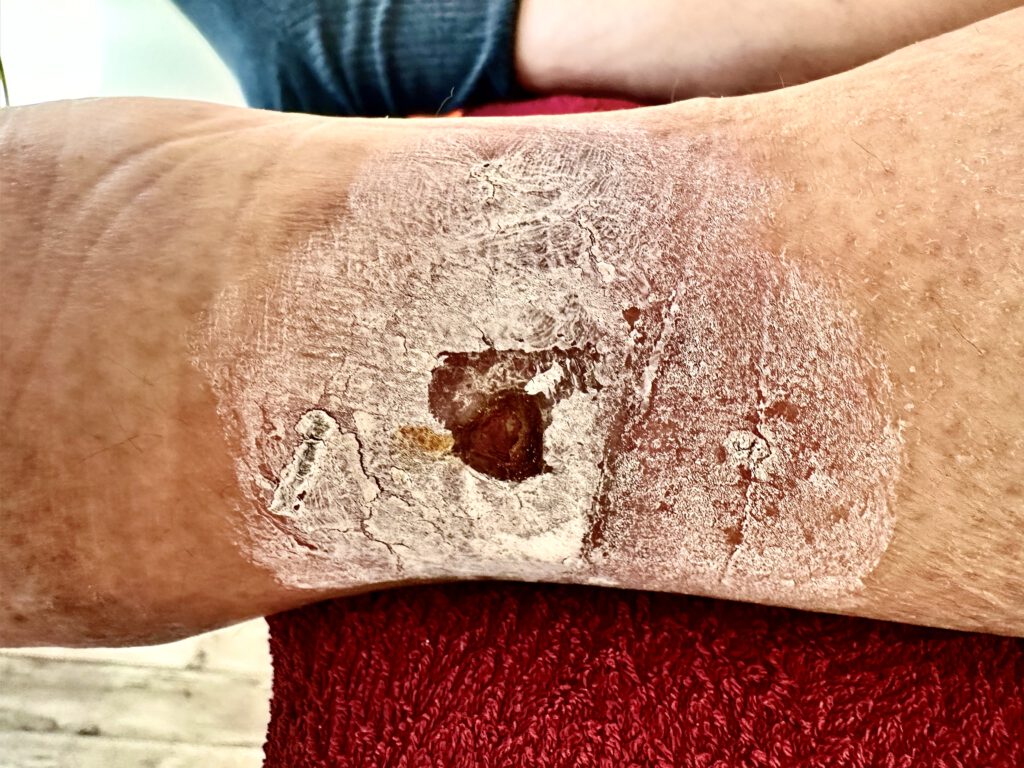
Saphenion®: long-term results of vein glue therapy for extension leg ulcer
The treatment of the so-called „open leg“ (ulcus cruris) has become much more straightforward and effective. Vein glue can also be used minimally invasively for the most severe findings. So far, we have used venous glue in 77 patients with leg ulcers, with great success!
O’Banion LA, Reynolds KB, Kochubey M, Cutler B, Tefera EA, Dirks R, Kiguchi MM. Treatment of superficial venous reflux in CEAP 6 patients: a comparison of cyanoacrylate glue and radiofrequency ablation techniques. J Vasc Surg Venous Lymphat Disord. 2021 Jan 13:S2213-333X(21)00001-9. doi: 10.1016/j.jvsv.2020.12.082. Epub ahead of print. PMID: 33453440.
Park I. Successful use of VenaSeal system for the treatment of large great saphenous vein of 2.84 cm diameter. Ann Surg Treat Res. 2018 Apr;94(4):219-221. doi: 10.4174/astr.2018.94.4.219. Epub 2018 Mar 26. PubMed PMID: 29629358; PubMed Central PMCID: PMC5880981.

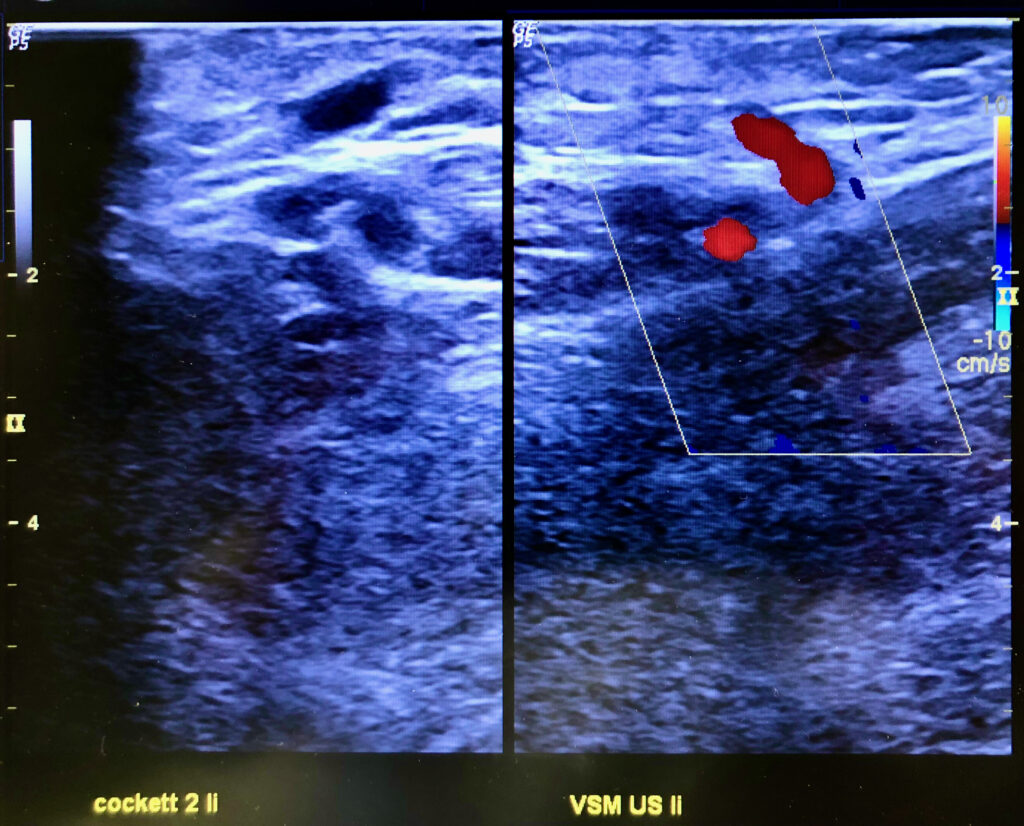
Saphenion: long-term results of vein glue – perforator veins
The human venous system has around 140 pairs of perforated veins, most of which are located on the lower leg. Of these connecting veins, about 18-20 pairs are of clinical importance, which is why they are also called key perforation veins. In over 60% of cases, these veins appear as a pair and accompany skin arteries as they pass through muscle fascia to the skin.
Therapy of perforating varicose veins with vein glue: This technique provides an elegant and safe way to close the perforating veins at the same time as the therapy of the truncal varicose veins using vein glue. On the one hand, perforation veins are already closed during the catheter maneuvre in the truncal vein by dispensing an additional drop of glue at the mouth (ultrasound control!). On the other hand, after sealing the saphenous veins, we also punctured and glued the perforating vein directly under sight in the same session with the puncture needle lying on the operating table.
Kiguchi MM, Reynolds KB, Cutler B, Tefera E, Kochubey M, Dirks R, Abramowitz SD, Woo EY, O’Banion LA. Perforator treatment is needed after VenaSeal and ClosureFast endovenous saphenous vein closure in CEAP 6 patients. J Vasc Surg Venous Lymphat Disord. 2021 Jun 7:S2213-333X(21)00295-X. doi: 10.1016/j.jvsv.2021.04.020. Epub ahead of print. PMID: 34111593.
Saphenion®: long-term results of vein glue – arterial occlusion
Saphenion has also started treating patients who suffer from an arterial occlusive disease (smoker’s leg) and functionally defective truncal varicose veins using VenaSeal® at the same time, or before the operation on the arteries, initially on the diseased veins. With this, a weighty dogma of vascular surgery – the importance of arterial therapy – could now be repealed!

Saphenion®: Long-term results of vein glue – outpatient therapy for seriously ill people
We now treat dementia or debilitating patients, Parkinson’s disease sufferers, and trisomy patients on an outpatient basis with light sedative medication. Systemic infectious diseases such as hepatitis, COVID-19 (66 cases), or HIV are also not a contraindication – patients with these underlying diseases have been successfully treated with the venous glue several times, without side effects.
Multiallergic diseases also have no contraindications for treatment with vein glue. Patients with lifelong thrombosis/embolism therapy can be successfully treated on an outpatient basis without discontinuing or changing the permanently necessary medication.
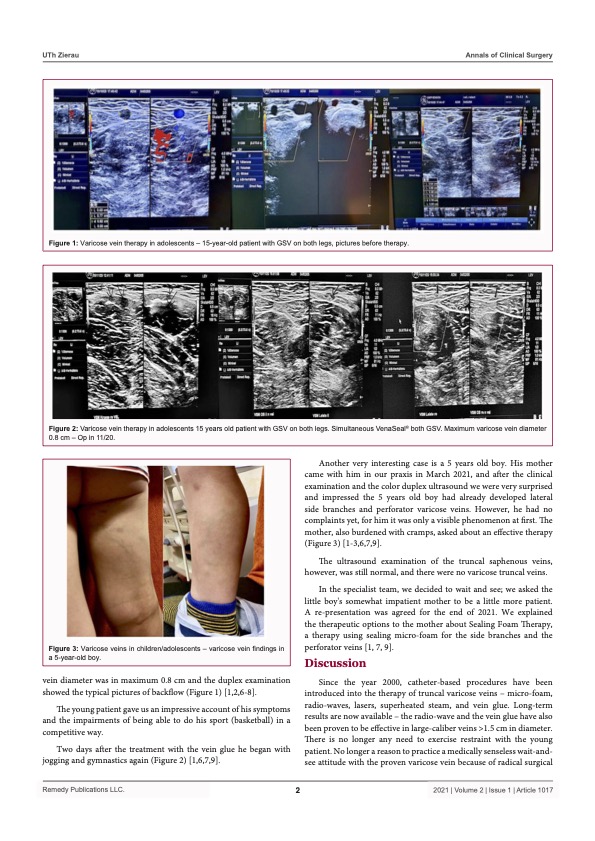
Saphenion®: Long-term results of vein glue therapy for adolescents
Saphenion®: 155 months vein glue – In the past, varicose vein therapy was practiced with great caution in children and adolescents. The well-known radical surgical „stripping“ – pulling out the trunk and side branch varicose veins was, in the imagination of the vascular surgeons, really unsuitable for working on the legs of young people, unless there was an acutely life-threatening finding (thrombosis) or a malformation (vascular tumor).
Since 2000, catheter-supported procedures have been introduced into the therapy of truncal varicose veins: microfoam, radio wave, laser, superheated steam, and vein glue. Long-term results are now available – the radio wave and the vein glue have also been proven to be effective in large-caliber veins > 1.5 cm in diameter. There is no more reason to exercise restraint with the young patient. There is no longer a reason to practice a medically senseless wait-and-see attitude with the proven varicose vein because of radical surgical techniques. This no longer heals, not with cream, gel, tablet, or even with compression stockings!
On the contrary, catheter procedures offer considerable advantages, especially for the young patient group, when considering radical surgical techniques‘ side effects and complications. And fully considering the end of the therapy options, the cold „non-thermal“ procedures, vein glue, and microform, are the best for the therapy of young patients. No anesthesia, no extensive anesthesia injections, and with the VenaSeal® vein glue, no more compression stockings. And we achieve immediate mobility for the adolescent patient.


Saphenion®: Long-term results of vein glue – pregnancy
Saphenion®: long-term results of vein glue – The classic therapy methods here are compression therapy with custom-made stockings or, better still, tights, manual lymph and tissue massages, and alternating showers, cold showers, and lots of exercises – midwives and obstetricians are very familiar with this.
http://www.babycenter.de/a8586/krampfadern
The situation is different with functionally defective side branches and truncal varicose veins. Incidentally, as is known from reticular and spider veins, these do not largely regress after delivery and weaning! There is a risk of serious complications, such as phlebitis or even thrombosis, especially during pregnancy (hormones and mechanical stress).
So far, the dogma has been that no active therapy methods are used to treat truncal varicosis during pregnancy!
Phlebitis is treated with compression therapy, administering antithrombotic drugs that do not flow through the placenta into the unborn child, and, if necessary, suctioning the thrombosed blood from the inflamed vein. Under certain circumstances, however, active therapy on the truncal varicose veins may also be essential from a prophylactic point of view. This must not result in anesthesia or large-scale injections of local anesthetics, as these drugs also reach the fetus via the placenta. A small local anesthetic must be enough! Conventional methods, stripping, and phlebectomy are eliminated, and lasers and radio waves are not feasible without general anesthesia or tumescence.
In clinical use for 13 years, we now have a catheter-supported procedure with the necessary criteria for use during pregnancy, vein gluing using VenaSeal® – Closure. Anesthesia is no longer necessary; the glue cannot cross the placenta after extensive tests by the American Health Authority, FDA, and the pregnant woman is in no way immobilized after the minimally invasive catheter operation.
Under strict consideration of the indications for this procedure – severe pain symptoms in the patient, phlebitis, and threatened thrombosis as well as severe infectious skin changes and ulcerations – this can be carried out after extensive information and the sole will of the patient.
Saphenion®: Long-term results of vein glue – side effects and complications
Our own experiences with the allergy to vein glue have recently been presented in an international publication:
A current search in the international specialist literature in preparation for this update has described the occurrence of contact dermatitis (inflammation of the skin after contact with cyanoacrylate) in 5 cases (in > 1,1 Mio patients!), and only one case was a supposed VenaSeal® allergy. Further complications during use or in the post-operative course are still not shown. We have not yet seen a VenaSeal® allergy, even after 2243 patients and 4716 veins were treated.
Almeida JI, Murray SP, Romero ME. Saphenous vein histopathology 5.5 years after cyanoacrylate closure. J Vasc Surg Venous Lymphat Disord. 2020 Mar;8(2):280-284. doi: 10.1016/j.jvsv.2019.04.014. Epub 2019 Jul 4. PMID: 31281102.
Fiengo L, Gwozdz A, Tincknell L, Harvey V, Watts T, Black S. VenaSeal closure despite an allergic reaction to n-butyl cyanoacrylate. J Vasc Surg Cases Innov Tech. 2020;6(2):269-271. Published 2020 Apr 10th. doi:10.1016/j.jvscit.2020.03.011
Jones AD, Boyle EM, Woltjer R, Jundt JP, Williams AN. J Vasc Surg Cases Innov Tech. 2019 Aug 7;5(3):372-374. doi: 10.1016/j.jvscit.2019.05.004. eCollection 2019 Sep.PMID: 31440717
Navarro-Triviño FJ, Cuenca-Manteca J, Ruiz-Villaverde R. Allergic contact dermatitis with systemic symptoms caused by VenaSeal. Contact Dermatitis. 2020;82(3):185-187. doi:10.1111/cod.13431
Park I. Human Saphenous Vein Histopathology 2 Years After Cyanoacrylate Closure Using the VenaSeal™ System. Ann Vasc Surg. 2021 Feb;71:534.e17-534.e21. doi: 10.1016/j.avsg.2020.09.017. Epub 2020 Sep 16. PMID: 32949737.
Watts TJ, Thursfield D, Haque R. Allergic contact dermatitis caused by VenaSeal tissue adhesive. Contact Dermatitis. 2019;80(6):393-395. doi:10.1111/cod.13206

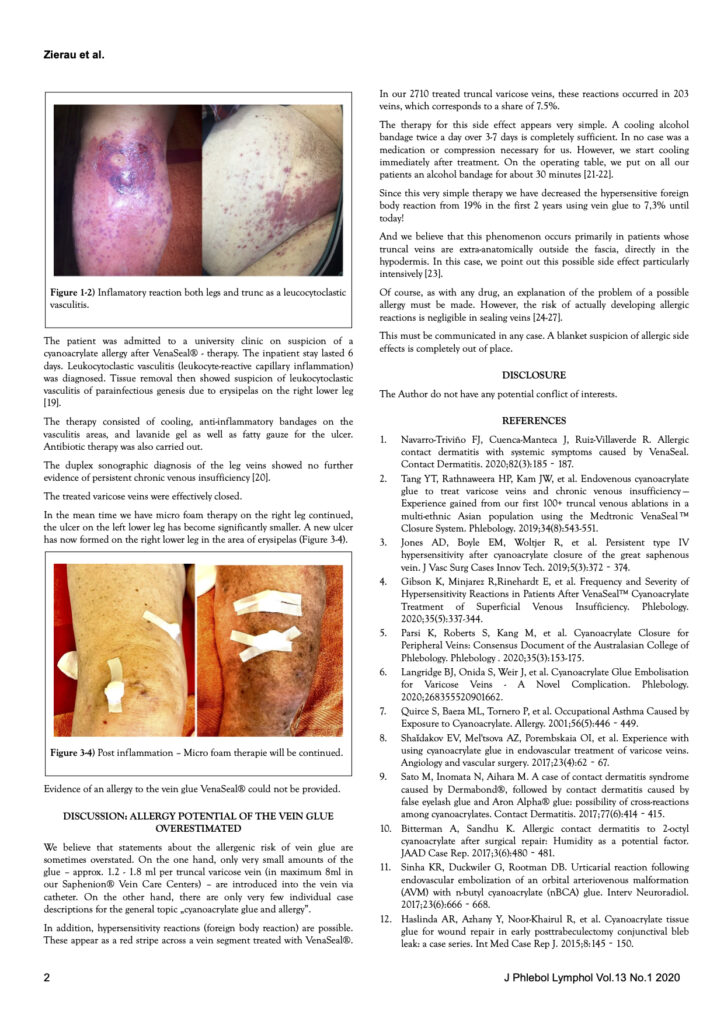

Our statement on alleged phlebitis after the vein glue – it is a tissue reaction of the surrounding tissue – has also been confirmed many times in further scientific studies:
Gibson K, Minjarez R, Rinehardt E, Ferris B. Frequency and severity of hypersensitivity reactions in patients after VenaSeal™ cyanoacrylate treatment of superficial venous insufficiency. Phlebology. 2020;35(5):337-344. doi:10.1177/0268355519878618
Nasser H, Ivanics T, Shakaroun D, Lin J. Severe phlebitis-like abnormal reaction following great saphenous vein cyanoacrylate closure. J Vasc Surg Venous Lymphat Disord. 2019 Jul;7(4):578-582. doi: 10.1016/j.jvsv.2019.03.010. Epub 2019 May 8. PubMed PMID: 31078516.
Park I, Jeong MH, Park CJ, Park WI, Park DW, Joh JH. Clinical Features and Management of „Phlebitis-like Abnormal Reaction“ After Cyanoacrylate Closure for the Treatment of Incompetent Saphenous Veins. Ann Vasc Surg. 2019;55:239-245. doi:10.1016/j.avsg.2018.07.040
Park I. Human Saphenous Vein Histopathology 2 Years After Cyanoacrylate Closure Using the VenaSeal™ System. Ann Vasc Surg. 2021 Feb;71:534.e17-534.e21. doi: 10.1016/j.avsg.2020.09.017. Epub 2020 Sep 16. PMID: 32949737.
Tang TY, Tiwari A. The VenaSeal™ Abnormal Red Skin Reaction: Looks Like but is not Phlebitis! Eur J Vasc Endovasc Surg. 2018;55(6):841. doi:10.1016/j.ejvs.2018.02.003
In a large American meta-analysis, all results and experiences on VenaSeal® therapy published by us and presented in our news were confirmed:
Kolluri R, Chung J, Kim S, Nath N, Bhalla BB, Jain T, Zygmunt J, Davies A. Network meta-analysis to compare VenaSeal with other superficial venous therapies for chronic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 2020 Feb 13. PII: S2213-333X(19)30702-4. doi: 10.1016/j.jvsv.2019.12.061. [Epub ahead of print] Review. PubMed PMID: 32063522.
Saphenion®: long-time results of vein glue – Discussion with Prof. Dr. Thomas Bürger about the results of vein glue
Saphenion®: Long-time results of vein glue – 155 months of therapy experience
Based on the 155 months of practical experience with 4716 treated saphenous veins in 2243 patients, we would like to confirm from our clinical and professional point of view for the fact check:
The vein glue is fully biocompatible, and so far, after more than 12 years of use (155 months), we have not seen any allergies. Two works in the literature describe a contact allergy of the skin after touching the glue. Allergies after injection into the varicose veins have only been described in one case worldwide.
The rate of side effects is, therefore, well below that of comparable thermal endovenous techniques (laser, radio wave) in treating truncal varicose veins.
The bioresorption of the glue takes between 10 and 36 months. The same resorption processes can also be found in humans as in the more than 150 publications on bioresorption in animals. The resorption models in animal experiments all show a biological degradation of the cyanoacrylate glue between 4 and 9 months. The absorption of the vein glue can also be followed macroscopically with regular ultrasound controls.
Furthermore, we now have our histological findings from human veins 10 – 14 months after endovenous cyanoacrylate vein glue. All four cases examined show a complete breakdown of the glue in the remnants of the vein. And here, too, in addition to our results, there is now another publication:
Almeida JI, Murray SP, Romero ME. Saphenous vein histopathology 5.5 years after cyanoacrylate closure. J Vasc Surg Venous Lymphat Disord. 2019 Jul 4. PII: S2213-333X(19)30325-7. doi: 10.1016/j.jvsv.2019.04.014. [Epub ahead of print] PubMed PMID: 31281102.
Saphenion®: Long-term results of vein glue therapy on the lower leg veins, risk of recurrence
Thermal therapy (laser, radio wave, superheated steam) on the lower leg has now been put in a critical light internationally and nationally, since the number of nerve lesions after thermal therapy cannot be neglected. At the annual meeting of the Bonner Venetage on the 8th of March 2024 in Bonn, this topic was discussed openly again. As a result, the majority took a critical stance on thermal therapy of the small saphenous vein, the great saphenous vein on the lower leg, and the therapy of perforating veins using laser or radio waves. These reservations now also apply to the treatment of recurrent varicose veins after radical surgical stripping.
Many colleagues now recommend doing without the thermal laser and radio wave methods; alternatively, catheter-supported microfoam therapy or vein glue is possible. Based on our experience and our study on over 2000 patients, we have defined an indication for microfoam or VenaSeal®. We treat truncal varicose veins on the lower leg with a diameter of up to 0.45 cm with microfoam, and truncal veins with a larger diameter are treated with vein glue.
Our alternative recommendation for radio waves for the saphenous veins on the lower leg, as previously stated in our educational discussions, is no longer applicable. We are now treating the lower leg exclusively with non-thermal methods.
This procedure was also anchored in the „Current guidelines for the therapy of varicosis“ of the DGP.
Saphenion®: Long-term results of vein glue – varicose vein aneurysm
Also, in aneurysmatic varicose veins with a diameter of 2,8 cm, VenaSeal® vein glue is effective and safe in therapy.
Saphenion®: Long-term results of vein glue – spider veins and reticular veins?
Saphenion®: Long-term results of vein glue – The vein glue for varicose veins is not, as can be read here and there and requested, suitable for the therapy of cosmetically disturbing reticular and spider veins. The microfoam, which has been the „gold standard“ since 2010, is available as a combination therapy.
A simultaneous double therapy with microfoam (ethoxysklerol) is possible but carries the risk of phlebitis. Scientific studies on this combination therapy have been submitted by Gibson et al.. It is essential first to wait for the truncal varicose veins to heal – in many cases, there is a significant decrease in the lateral branch varicosis! This fact is also considered with Saphenion®, and we only carry out the necessary microfoam therapies 14 – 21 days after the VenaSeal® therapy.
Photo / Video: Claudia, Madleen, Utzius
Papers / Links
Copy of page 11 of the FDA approval for the biocompatibility of the VenaSeal® vein adhesive:
Table 4: Results of Biocompatibility Testing – VenaSeal Adhesive (Polymerized and the Unpolymerized States) Test Method Reference Results Cytotoxicity (Elution Method) ISO 10993-5.
The cumulative results of the VenaSeal adhesive material cytotoxicity testing, in combination with assessments of toxicological risk and in vivo use, support an overall favorable cytotoxicity profile for the VenaSeal adhesive material per its intended use.
ISO MaximizationSensitization Study(Guinea Pigs)ISO 10993-10VenaSeal adhesive does not elicit a sensitization response
ISO Intracutaneous Reactivity – ISO 10993-10: The cumulative results support that the VenaSeal® adhesive material does not cause intracutaneous reactivity (Material Mediated Rabbit Pyrogenicity
ISO 10993-5US Pharmacopeia Section 151: The cumulative results support that the VenaSeal adhesive material is non-pyrogenic.
Acute Systemic Toxicity- ISO 10993-11: The cumulative results support that the VenaSeal adhesive material is not considered to cause acute systemic toxicity
Subacute / Subchronic ToxicityImplantation (13 weeks) – ISO 10993-11 / ISO 10993-6: The cumulative results support that the VenaSeal adhesive material does not result in any specific adverse systemic toxicological findings in the tissues examined. Genotoxicity (Bacterial Mutagenicity, in vitro Mouse Lymphoma Assay, Mouse Micronucleus Assay)
ISO 10993-3: The cumulative results support that the VenaSeal adhesive material is non-mutagenic Hemo-compatibility (Hemolysis, Complement Activation, Partial Thromboplastin Time, Platelet and Leukocyte Count), ASTM F-756-08
ISO 10993-4: The cumulative results support that the VenaSeal adhesive material is non-hemolytic and does not cause chronic toxicity upon Implantation (26 Weeks)
ISO 10993-11/ ISO 10993-6: The cumulative results support that VenaSeal – adhesive does not cause any significant adverse systemic or local toxicity in the tissues examined.
Quelle: Summary – Food and Drug Administration / FDA
Almeida JI, Murray SP, Romero ME. Saphenous vein histopathology 5.5 years after cyanoacrylate closure. J Vasc Surg Venous Lymphat Disord. 2020 Mar;8(2):280-284. doi: 10.1016/j.jvsv.2019.04.014. Epub 2019 Jul 4. PubMed PMID: 31281102.
Bahi M, Guazzo L, Taumoepeau L. Real-world short-term VenaSeal ablation outcomes for symptomatic saphenous incompetence. Vascular. 2023 Jun;31(3):521-525. doi: 10.1177/17085381221077511. Epub 2022 Feb 25. PMID: 35209758.
Bozkurt AK, Balkanay OO, Dinc R. Comparative analysis of VenaBlock and VenaSeal Systems for catheter-guided endovenous cyanoacrylate closure in treating chronic venous insufficiency of the lower extremity: effectiveness and feasibility. Int Angiol. 2024 Jun;43(3):331-341. doi: 10.23736/S0392-9590.24.05143-5. Epub 2024 Jul 23. PMID: 39041783.
Chan YC, Law Y, Cheung GC, Ting AC, Cheng SW. Cyanoacrylate glue used to treat great saphenous reflux: Measures of the outcome. Phlebology. 2017 Mar;32(2):99-106. doi: 10.1177/0268355516638200. Epub 2016 Jul 9. PubMed PMID: 27052039.
Chan SSJ, Yap CJQ, Tan SG, Choke ETC, Chong TT, Tang TY. The utility of endovenous cyanoacrylate glue ablation for incompetent saphenous veins in the setting of venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2020 Mar 20. PII: S2213-333X(20)30100-1. doi: 10.1016/j.jvsv.2020.01.013. [Epub ahead of print] PubMed PMID: 32205130.
Cho S, Park HS, Lee T, Byun SJ, Yun WS, Yang SS, Kim H, Kim WS, Joh JH, Jung IM. CASS (CyanoAcrylate closure versus Surgical Stripping for incompetent saphenous veins) study is a randomized controlled trial comparing clinical outcomes after cyanoacrylate closure and surgical stripping for treating incompetent saphenous veins. Trials. 2020 Jun 3;21(1):460. doi: 10.1186/s13063-020-04393-0. PMID: 32493398; PMCID: PMC7268719.#
Dinc R. VenaBlock® and VenaSeal® class III cyanoacrylate products are effective and safe in varicose vein treatment. Phlebology. 2024 May;39(4):284-285. doi: 10.1177/02683555231221319. Epub 2023 Dec 10. Erratum in: Phlebology. 2024 Dec;39(10):723. doi: 10.1177/02683555241264093. PMID: 38073234.
Gibson K, Ferris B. Cyanoacrylate closure of incompetent great, small, and accessory saphenous veins without the use of post-procedure compression: Initial outcomes of a post-market evaluation of the VenaSeal System (the WAVES Study). Vascular. 2017 Apr;25(2):149-156. doi: 10.1177/1708538116651014. Epub 2016 Jul 9. PubMed PMID: 27206470.
Gibson K, Morrison N, Kolluri R, Vasquez M, Weiss R, Cher D, Madsen M, Jones A. Twenty-four month results from a randomized cyanoacrylate closure versus radiofrequency ablation trial for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2018 Sep;6(5):606-613. doi: 10.1016/j.jvsv.2018.04.009. Epub 2018 Jun 15. PubMed PMID: 29914814.
Jones AD, Boyle EM, Woltjer R, Jundt JP, Williams AN.J Vasc Surg Cases Innov Tech. 2019 Aug 7;5(3):372-374. doi: 10.1016/j.jvscit.2019.05.004. eCollection 2019 Sep.PMID: 31440717
Jones AD, Boyle EM, Woltjer R, Jundt JP, Williams AN. Persistent type IV hypersensitivity after cyanoacrylate closure of the great saphenous vein. J Vasc Surg Cases Innov Tech. 2019 Aug 7;5(3):372-374. doi: 10.1016/j.jvscit.2019.05.004. eCollection 2019 Sep. PubMed PMID: 31440717; PubMed Central PMCID: PMC6699189.
Kiguchi MM, Reynolds KB, Cutler B, Tefera E, Kochubey M, Dirks R, Abramowitz SD, Woo EY, O’Banion LA. The need for perforator treatment after VenaSeal and ClosureFast endovenous saphenous vein closure in CEAP 6 patients. J Vasc Surg Venous Lymphat Disord. 2021 Jun 7:S2213-333X(21)00295-X. doi: 10.1016/j.jvsv.2021.04.020. Epub ahead of print. PMID: 34111593.
Kolluri R, Chung J, Kim S, Nath N, Bhalla BB, Jain T, Zygmunt J, Davies A. Network meta-analysis to compare VenaSeal with other superficial venous therapies for chronic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 2020 Feb 13. PII: S2213-333X(19)30702-4. doi: 10.1016/j.jvsv.2019.12.061. [Epub ahead of print] Review. PubMed PMID: 32063522.
Lim C, Hsu J, Vo T, Behseresht J, Tayyarah M, Andacheh I. A Comparison of Venaseal Versus Radiofrequency Ablation Outcomes Within a Managed Care Organization. Ann Vasc Surg. 2024 Feb;99:75-81. doi: 10.1016/j.avsg.2023.09.088. Epub 2023 Nov 10. PMID: 37952570.
Nasser H, Ivanics T, Shakaroun D, Lin J. Severe phlebitis-like abnormal reaction following great saphenous vein cyanoacrylate closure. J Vasc Surg Venous Lymphat Disord. 2019 Jul;7(4):578-582. doi: 10.1016/j.jvsv.2019.03.010. Epub 2019 May 8. PubMed PMID: 31078516.
Navarro-Triviño FJ, Cuenca-Manteca J, Ruiz-Villaverde R. Allergic contact dermatitis with systemic symptoms caused by VenaSeal. Contact Dermatitis. 2020 Mar;82(3):185-187. doi: 10.1111/cod.13431. Epub 2019 Nov 15. PubMed PMID: 31674037.
Park I. Successful use of VenaSeal system for the treatment of large great saphenous vein of 2.84 cm diameter. Ann Surg Treat Res. 2018 Apr;94(4):219-221. doi: 10.4174/astr.2018.94.4.219. Epub 2018 Mar 26. PubMed PMID: 29629358; PubMed Central PMCID: PMC5880981.
Park I. Initial Outcomes of Cyanoacrylate Closure, VenaSeal System, for the Treatment of the Incompetent Great and Small Saphenous Veins. Vasc Endovascular Surg. 2017 Nov;51(8):545-549. doi: 10.1177/1538574417729272. Epub 2017 Oct 2. PubMed PMID: 28969499.
Park I, Kim D. Correlation Between the Immediate Remnant Stump Length and Vein Diameter After Cyanoacrylate Closure Using the VenaSeal System During Treatment of an Incompetent Great Saphenous Vein. Vasc Endovascular Surg. 2019 Oct 3:1538574419879563. doi:10.1177/1538574419879563. [Epub ahead of print] PubMed PMID: 31581906.
Shaĭdakov EV, Mel’tsova AZ, Porembskaia OI, Kudinova EA, Korzhevskiĭ DÉ, Kirik OV, Sukhorukova EG. [Experience with using cyanoacrylate glue in endovascular treatment of varicose veins]. Angiol Sosud Khir. 2017;23(4):62-67. Russian. PubMed PMID: 29240057.
Lam YL, De Maeseneer M, Lawson J, De Borst GJ, Boersma D. Expert review on the VenaSeal® system for endovenous cyanoacrylate adhesive ablation of incompetent saphenous trunks in patients with varicose veins. Expert Rev Med Devices. 2017 Oct;14(10):755-762. doi: 10.1080/17434440.2017.1378093. Review. PubMed PMID: 28892412.
Lane TR, Kelleher D, Moore HM, Franklin IJ, Davies AH. Cyanoacrylate glue for the treatment of great saphenous vein incompetence in the anticoagulated patient. J Vasc Surg Venous Lymphat Disord. 2013 Jul;1(3):298-300. doi: 10.1016/j.jvsv.2012.09.007. Epub 2013 Feb 15. PubMed PMID: 26992590.
Morrison N, Gibson K, McEnroe S, Goldman M, King T, Weiss R, Cher D, Jones A. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). J Vasc Surg. 2015 Apr;61(4):985-94. doi: 10.1016/j.jvs.2014.11.071. Epub 2015 Jan 31. PubMed PMID: 25650040.
Morrison N, Gibson K, Vasquez M, Weiss R, Jones A. Five-year extension study of patients from a randomized clinical trial (VeClose) comparing cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2020 Mar 20. PII: S2213-333X(20)30105-0. doi: 10.1016/j.jvsv.2019.12.080. [Epub ahead of print] PubMed PMID: 32205125.
O’Banion LA, Reynolds KB, Kochubey M, Cutler B, Tefera EA, Dirks R, Kiguchi MM. Treatment of superficial venous reflux in CEAP 6 patients: a comparison of cyanoacrylate glue and radiofrequency ablation techniques. J Vasc Surg Venous Lymphat Disord. 2021 Jan 13:S2213-333X(21)00001-9. doi: 10.1016/j.jvsv.2020.12.082. Epub ahead of print. PMID: 33453440.
Park I. Human Saphenous Vein Histopathology 2 Years After Cyanoacrylate Closure Using the VenaSeal™ System. Ann Vasc Surg. 2021 Feb;71:534.e17-534.e21. doi: 10.1016/j.avsg.2020.09.017. Epub 2020 Sep 16. PMID: 32949737.
Tang TY, Yap CJQ, Chan SL, Soon SXY, Yap HY, Lee SQW, Choke ETC, Chong TT. Early results of an Asian prospective multicenter VenaSeal real-world postmarket evaluation to investigate the efficacy and safety of cyanoacrylate endovenous ablation for varicose veins. J Vasc Surg Venous Lymphat Disord. 2021 Mar;9(2):335-345.e2. doi: 10.1016/j.jvsv.2020.03.020. Epub 2020 May 7. PMID: 32387378.
Watts TJ, Thursfield D, Haque R. Allergic contact dermatitis caused by VenaSeal tissue adhesive. Contact Dermatitis. 2019 Jun;80(6):393-395. doi: 10.1111/cod.13206. Epub 2019 Jan 30. PubMed PMID: 30582174.
Zierau UT and Lahl W: Recurrence Discussion in Varicose Veins Therapy – A Critical Examination of the Vein Stump discussion; J. Vasc. Endovasc. Therapy 2019, Vol 4 No.2:13
https://www.saphenion.de/news/konsensus-1-neef-endovenoese-krampfadertherapie/embed/#?secret=kPFV94HWjphttps://vascularnews.com/veclose-venaseal-safe/embed/#?secret=5Wfx465gUI

